
The History of GRAS
When the food additive amendments to the Food, Drug, and Cosmetic Act (FDCA) were enacted in 1958, certain food ingredients with a long history of use were exempted from the pre-market evaluation and approval process required for food additives. Such compounds were labeled as “generally recognized as safe” (GRAS) under the conditions of their intended use. However, any food ingredient can be considered GRAS—as long as it is generally recognized among scientific experts (qualified by scientific training and experience) to be safe under the conditions of its intended use.
The GRAS status of a compound could be established either through submission of a GRAS petition to the FDA or through a GRAS self-determination, which was not formally submitted to FDA. When the FDA approved a GRAS petition, the ingredient was listed in the Code of Federal Regulations (CFR). When a company made a GRAS self-determination for an ingredient, it retained all the documentation, making it available, as needed, for marketing or regulatory purposes.
Part of the proposed rule clarified requirements for determining the GRAS status of a food ingredient. Key elements were: (1) evidence of the ingredient's safety, and (2) a basis to conclude that this evidence is generally known and accepted. Technical evidence uses one or two approaches: (1) scientific procedures or (2) common use in food prior to January 1, 1958.
The new notification procedure also specified both the format and scientific content of the submission to FDA. Notification was not mandatory, but was to be available if the sponsor (i.e., the company desiring GRAS status for the ingredient) wished to inform FDA of its GRAS determination. The FDA indicated in the April 17, 1997, notice that it would no longer accept GRAS petitions. This meant that, other than Flavor and Extract Manufacturers Association (FEMA) GRAS ingredients, all future GRAS determinations would rely strictly on self-determinations, and manufacturers may or may not notify FDA of such a determination.
Current GRAS Compounds
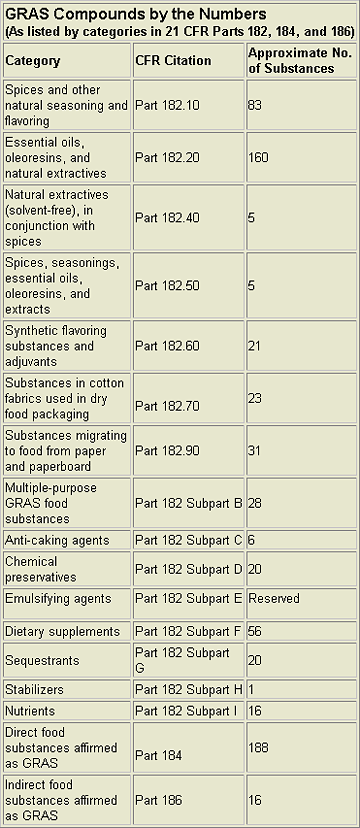
The FDA officially lists compounds it considers GRAS in 21 CFR Parts 182, 184, and 186. Over 680 substances are given. (See the chart, “GRAS Compounds by the Numbers.”) However, the list does not include every GRAS substance. The FDA realizes that it is impractical to list all such items, and emphasizes this is just a fraction of the compounds that can be considered GRAS. For example, in addition to these 680+ compounds, FEMA has independently affirmed at least another 1,000 compounds as GRAS. The FDA accepts the FEMA GRAS review process as consistent with the criteria set forth in the FDCA and has, essentially, adopted the FEMA list as a de facto official FDA-approved list of GRAS flavoring agents.Additionally, there have been 97 self-affirmation GRAS notifications submitted to FDA through Jan. 2002 (see “Website Resources” at end of this article). Of these, 56 have received “no objection” letters, 25 were rejected or withdrawn, 7 were resubmitted, and 16 are still pending.
Overall, most FDA-approved compounds are “indirect additives” used in paper and plastic packaging that may migrate into the food itself. These indirect additives number in the thousands and are listed in 21 CFR, parts 174-178.
In contrast, a relatively small number of compounds are approved for direct addition to food. About 280 direct food additives and 55 color additives are approved by the FDA under 21 CFR Parts 73, 74, 75, 172 and 173. An additional 880+ synthetic and natural flavorings are approved under 21 CFR part 172.510 and 172.515.

Most originally-listed substances have been reaffirmed as GRAS by SCOGS. However, 6% of the original substances are conditionally approved, with additional research recommended (examples include carrageenan, oil of nutmeg, glutamates and caffeine). Another 8% are judged to have insufficient data available to affirm safety. For these substances, the FDA has requested additional data from industry and will rescind their GRAS status if it does not appear.
Most chemicals directly added to food are GRAS. See the chart “Typical GRAS Substances” for examples. GRAS substances represent a highly-diverse group of chemical structures with diverse toxicological characteristics. Most are present because they have a long history of use without significant reports of adverse health effects. The FDA cannot remove an agent from the GRAS list unless evidence appears showing the substance is no longer safe for its intended use.
The levels of addition have been specified for only some substances. For most, usage levels are defined according to the amounts consistent with “good manufacturing practice.” In cases where the usage limit of a substance has been determined, new uses that result in an increased intake of the substance by consumers must be justified, based on scientific procedures.
It is difficult to generalize about the criteria used to affirm or judge a food additive as GRAS. Expert judgment is an important part of the process. However, the quantity and quality of the available toxicology data for each of the substances on the list vary greatly, and the decisions made by experts cannot readily be shown to rest on a particular set of criteria. Therefore, it is not possible to set out a list of toxicological results that must be available before a substance can be listed as GRAS. It is clear, however, that whenever new uses of a GRAS substance are contemplated, the scientific experts conducting the review usually will consider whether new toxicology studies are to be conducted to ensure continued GRAS status. Most importantly, the pivotal safety studies must be published and available for peer review.
What Does It Mean?
In the past, most food companies would only use ingredients specifically approved for use in food in the Code of Federal Regulations (CFR). However, there has been a dramatic decrease in new CFR-listed food additives. This is due to a dramatic increase in GRAS determination and subsequent notifications; there has been an equally dramatic decrease in new food additive petitions since 1997. Thus, fewer and fewer CFR-listed food additives are available for development of new food products.It should be emphasized, however, that this does not mean there are fewer ingredients available for product development. In fact, there have been over 55 “successful” GRAS food notifications to date. In addition to these 55, there probably have been at least another 100 ingredients that have GRAS self-determinations not submitted to FDA. Clearly, most innovative food companies probably have been using new GRAS ingredients in products developed over the last five years.
GRAS ingredients are not new, but their recent ascendance as the main source of food ingredient innovation is something that cannot be ignored, especially as the food industry moves into the 21st century.
Links
- FDA Center for Food Safety and Applied Nutrition
- Guidance and reference documents for petitions and notifications
- Federal Register Proposed GRAS Notification Procedures, April 17, 1997
- Self-affirmed GRAS list
- Inventory of all food additive and GRAS ingredients listed in the 21 CFR
- Inventory of GRAS notifications