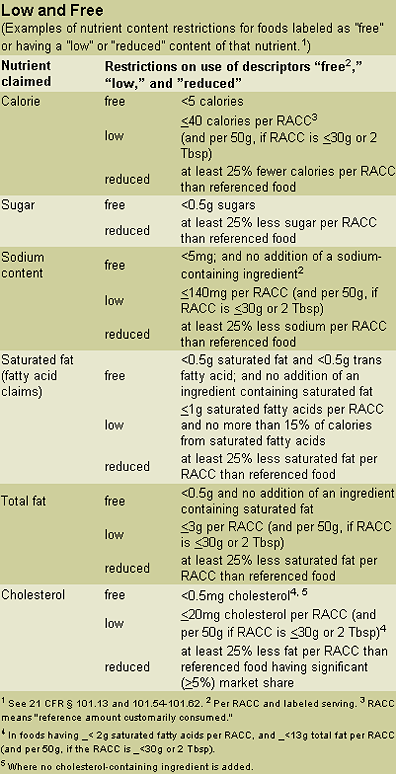
Nutrient content. A nutrient content claim focuses on the level of a nutrient in a product. Specific claims—based on analysis—may be made for calorie content, sugar, sodium, total fat, saturated fat (fatty acid claims), and cholesterol. Other criteria also may be required. (See “Website Resources” at end.)
Nutrient content claims may be made in either an “explicit” or “implied” format. An implied nutrient content claim is a statement that “suggests” the absence or presence of a particular nutrient; e.g., “good source of calcium” suggests a high calcium content, “high in oat bran” denotes a high fiber content, and “no tropical oils” indicates absence of these oils.

Not all statements about nutrients are “nutrient content claims,” however. For example, statements such as “no preservatives,” “no artificial colors,” and “no caffeine” are statements of fact referring to substances that have no nutritive value. Similarly, other statements about the absence of an ingredient are used to help consumers avoid that ingredient because of allergies, religious beliefs or dietary preferences.
When making a nutrient content claim, the manufacturer may need to disclose when certain nutrients exceed specified levels, and this disclosure depends upon the product and serving size (see 21 CFR § 101.13). One example of such disclosure is the statement, “See side panel for information about total fat and other nutrients.” Foods generally trigger the disclosure requirement when they contain more than 13.0g total fat, 4.0g saturated fat, 60mg cholesterol, or 480mg sodium per the reference amount customarily consumed (RACC) and the labeled serving size. Main dishes or meal products have different disclosure requirements for these nutrients.
What must a food company know before making a nutrient content claim? The company must be careful to use only those terms or descriptors defined and designated by the regulations. For example, a food product may be labeled as “sugar free,” provided it contains no more than 0.5g of sugar per RACC. If the sugar content exceeds that level, the FDA would likely find the food product misbranded.
Health Claims. Sometimes referred to as “disease-prevention claims,” health claims are statements made on the label of a food product, or a dietary supplement, that characterize the relationship of a substance to a disease or health-related condition other than a nutritional deficiency, such as scurvy. The substance can be a food or a food component, whether the food is a conventional food product or a dietary supplement, such as vitamins or minerals.
Health claims may be obtained by petitioning the FDA with the company's own data which substantiates the proposed claim. Alternatively, the company may rely on an authoritative statement from a governmental agency.
When making a health claim, the food company needs to comply with the regulations. Otherwise, it risks the food product being deemed misbranded. 21 CFR § 101.14 and 101.70 set forth general principles of health claims and outline procedures for petitioning the FDA to allow a specific health claim.
A food product can be disqualified from having a health claim if it contains fat (total or saturated), cholesterol, or sodium at a level exceeding the maximum level permitted for the nutrient. The “disqualifying” levels are specified in 21 CFR § 101.14(a). If the labeled food exceeds one or more of these levels, a health claim cannot be made, unless FDA provides for a specific exception. (See Disqualifying Nutrients chart.)
Health claims must also meet FDA's “jelly bean” rule, so named to prevent health claims on products void of any nutritional value. Under this rule, prior to any nutrient addition, the food must contain at least 10% of the daily recommended value of at least one of Vitamin A, Vitamin C, calcium, iron, protein, or fiber per RACC. Fresh fruits and vegetables are exempt from this requirement.

The FDA currently authorizes 13 health claims. One example of such a claim for calcium as it relates to osteoporosis is, “Regular exercise and a healthy diet with enough calcium helps teens and young white and Asian women maintain good bone health and may reduce their high risk of osteoporosis later in life.”

Structure/Function Claims. Authorized by the Dietary Supplement Health and Education Act (DSHEA) of 1994, a structure/function claim describes a product's ability to affect the structure or function of the human body, including general well-being, from consumption of the nutrient. Three key points are important here: substantiating the claim with evidence, avoiding the use of names of the disease or condition in the claim, and including a disclaimer, discussed below.
To make a structure/function claim, the company must have evidence substantiating the claim. It need not seek pre-market approval, which is required for nutrient and health claims that are not based on an authoritative statement. The company must, however, notify FDA of making the claim within 30 days of initially marketing the product.
In terms of phrasing, the same precautions applying to health claims come into play with structure/function claims. The claim must be carefully worded to avoid becoming a drug claim. To avoid drug status, phrase the claim so it does not refer to the disease in phrases such as “protects against osteoporosis,” or “protects against development of coronary heart disease.” Acceptably phrased claims focus, instead, on the relief of symptoms or the maintenance of normal functions, such as “reduces stress and frustration” and “helps maintain healthy cholesterol levels.” Another approach involves describing the general well-being attributed to the consumption of the nutrient or the dietary supplement.
Additionally, the label must include a disclaimer that reads, “This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.”
Although structure/function claims may be used to highlight a dietary supplement or an ingredient in a food product, a disparity exists in the labeling regulations governing foods and dietary supplements. The regulations allow ingredients that are not GRAS or prior sanctioned (such as botanicals), to be used in dietary supplements and their presumed health benefits played up by structure/function claims. For, example, dietary supplements containing St. John's wort or Echinacea can lawfully use structure/function claims to tout the benefits of these constituents. However, when used in a food product, the same non-approved ingredients render the food adulterated.
Numerous options are available for marketers and food research professionals to convey nutrition information about their products to consumers, but care must be exercised to ensure the information meets regulatory requirements.