
The addition of functional acidulants, the removal of fat, or the use of alternative sweeteners in a food product can result in an undesirable tartness. The wish to market unconventionally flavored foods, such as chocolate yogurt, makes sourness a problem in otherwise traditional products.
Astringency, aftertaste and mouthfeel complicate the perception of an acid. Though there are few rules of thumb, a checklist of options offers suggestions to control sourness.
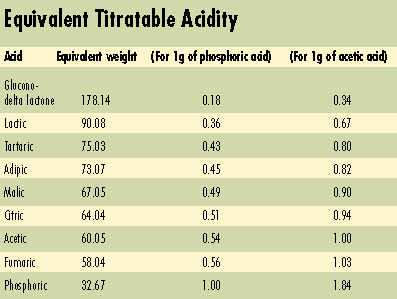
Accomplished Acidifiers
Acids, which are naturally present in fruits and vegetables, find their way into formulas for many reasons. Many contribute to a great tasting product: the tartness of citrus products or the tang of vinegar, for example. Fermentation of foods by microbes results in lactic, acetic and other acids.Other acidifiers are added for functional reasons. Acids lower pH and heighten the effectiveness of preservatives—such as the organic acids sodium benzoate or potassium sorbate—for increased microbial stability. And oxidation is inhibited by reducing agents such as ascorbic and erythorbic acid or by chelating acids including citric acid and EDTA.
Reducing a product's pH also helps minimize the amount of heat needed for processing, which in turn protects the texture and flavor of foods. Food-grade acids also perform as leavening agents, improve foam and color stability, inactivate degradation enzymes, function as anticoagulants, and aid in gel formation and the curing of meat.
When the tartness of these functional ingredients gives a pronounced and undesirable sour taste, formulators may consider a series of approaches.

Checklist to Tame Tartness
1. Balance the sweet/sour profile with sugars. This is often the first suggestion given. Increasing sugar negates some of the acidity. Corn syrups mask tartness more than sucrose, but with a different flavor profile. Additionally, corn syrup solids and high fructose corn syrup have pH levels in the 3.5 to 5.0 range and may themselves help lower the pH of products such as pie fillings.2. Use acid blends to smooth intensity peaks. The perception of how intense an acidulant's sourness is will vary over time. Citric acid, which gives a burst of tartness, can be combined with malic or adipic acid to smooth the taste sensation. Malic acid also has a sharp taste, but it comes on more slowly than citric acid. The taste of lactic acid is milder and tends to linger on.
3. The perceived tartness of an acidifier can change depending on other ingredients in the formula, an acidifier's concentration, the pH of the food or beverage and even product temperature. For example, one supplier of sodium acid sulfate (which may also be labeled as “bisulfate of soda” or “sodium bisulfate,” says sensory tests show it has a sour intensity greater than citric, phosphoric or malic acid when used at concentrations of roughly 0.06% or more in water. However, low concentrations of 0.001% to 0.0135% (at pH levels from 4.0 down to 3.0, respectively), sodium acid sulfate is perceived to be less sour than these three alternative acids.
4. Use acidifiers that are compatible with the flavors present. Examples include lactic acid with dairy products, or malic acid with apple flavors. Note, however, that acid ratios can shift in natural products. For example, the high-malic/low-tartaric acid ratio in grapes reverses itself as the grape ages—an important factor in wine quality.
5. Add a second flavor component that is commonly associated with an acid tang. Strawberry flavor in vanilla yogurt, sour cream in a chocolate dessert, barbecue sauce in meat, or wine flavor in cheese sauce result in products with acceptable sensory profiles even though the food is acidified for other reasons. Soy sauce, a widely used “flavor enhancer,” also has a slightly tart note associated with it.
6. Choose an acid with a mild flavor. Glucono-delta-lactone (GDL), which slowly hydrolyzes to gluconic acid, carries a reputation as an unusually mild acidulant; citric or fumaric acids more readily impart tartness.
7. Use buffers. Buffers, such as sodium citrate, are commonly used to control pH. However, highly buffered acid solutions appear to convey greater and more lingering sourness compared with unbuffered acids at the same pH.
8. Remove the need for the acid in the first place. High-methoxyl pectins require a low pH to produce a gel in candy, while low-methoxyl pectins only need calcium ions for gel formation. Blanching vegetables inhibits enzymatic activity as well or better than low pHs. Minimizing the microbial loads in salads may allow slightly higher pHs.
As formulators know, a touch of tanginess is tantalizing, but a striking sourness suppresses sales.
Website Resources
http://vm.cfsan.fda.gov/~comm/lacf-phs.html— A list of various foods' pHswww.uark.edu/depts/foodsci/mystery/extra/10_pH.html— Alkalinity and acidity of food
http://seafood.ucdavis.edu/HACCP/Compendium/Terms.html— Discussion of pHs
www.jungbunzlauer.com/products/product_15.html— Information on GDL
www.elc-eu.org/5.htm#2— A few comments on acidifier properties
Sidebar: Neutralizing Effects
“Alkaline food” is a hot topic in the natural products industry; just type “alkaline foods” into any Internet search engine. A food scientist would be bewildered by the foods—fruits for example—that are categorized as alkaline. One health guru explains that how a food is listed depends on whether the food's ash is alkaline or acidic and that what is needed is “alkaline-ash foods to offset the effect of acid-forming foods.” Seewww.inlighttimes.com/archives/2002/11/acid-alkaline-foods.htm.For food scientists trying to formulate finished products with low pHs (high titratable acidity) for microbial safety or other reasons, very few alkaline foods (as defined by those with pHs higher than 7.0) exist to thwart their effort by unintentionally buffering or neutralizing a food's acidic ingredients. Such reactions are not always instantaneous, such as in non-liquid foods, with the pH rising over time.
Of nearly 420 foods listed on one USDA website, only a dozen or less possess pHs that can range above 7.0. Egg whites check in with a very alkaline pH of almost 8.0. Cooked corn and spinach also lean toward pHs over 7.0.
The pH of phosphates vary from acidic (e.g., monosodium phosphate) to alkaline (e.g., tripotassium phosphate). Phosphates are commonly used on or in shrimp, surimi or other protein products to improve yields and texture. When the surimi is used in a mayonnaise or vinegar-based salad, the finished product's pH will rise. Most common alkaline foods are that way due to their processing. Corn tortillas (masa) are made with calcium hydroxide, black olives (pH 6.00 to 7.30) with sodium hydroxide. Other high-pH foods are soda crackers (5.65 - 7.32), Camembert cheese (7.44) and graham crackers (7.10 to 7.92).
 Claudia Dziuk O'Donnell is Chief Editor and Associate Publisher of Prepared Foods magazine including its NutraSolutions and Culinary sections. Her responsibilities include determining the editorial content of the print publication and the New Products Conference.
Claudia Dziuk O'Donnell is Chief Editor and Associate Publisher of Prepared Foods magazine including its NutraSolutions and Culinary sections. Her responsibilities include determining the editorial content of the print publication and the New Products Conference.