Acids, in themselves, affect sensory systems in four different ways. In the mouth, they impact taste receptor cells, which detect sourness, and the trigeminal system, which is sensitive to chemical irritation. Examples of the latter include extremely sour candies or the dryness or astringency from tannins and certain acids.
In the nasal cavity, aromas are detected by olfactory receptor cells while volatile chemical irritants, such as those associated with horseradish, affect the nasal trigeminal system. All acids have an oral impact but only some have a nasal impact.
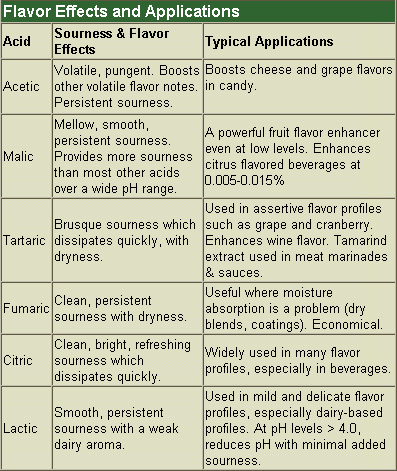
The amount of sourness is determined by which acid is used, its concentration and the pH. At any given pH, an equilibrium is reached between an acid's undissociated—or protonated—forms (e.g. lactic acid) and dissociated—or ionized—forms (e.g. lactate). As pH decreases, levels of the sour-tasting, undissociated forms substantially increase (completely dissociated acids are not sour).
Because the amount of an acid's undissociated forms at different pH levels can be calculated, so can the relative sourness between acids at different pH levels be estimated. For example, at or above pH 4.0, lactic acid is less sour than most acids; below 4.0, it is more sour than citric acid.
These sourness models are helpful in choosing acids for a desired perception of sourness at a specific pH. However, other factors also are important. For example, products that are mostly water, such as beverages, are very sensitive to changes in the acidic ingredients. Products containing higher amounts of hydrocolloids and fat are less sensitive.
A mutual masking between sweetness and sourness also exists. Some pigments, flavors, and gelling agents are very sensitive to pH—so a narrow pH range is needed for consistency. This is achieved through the use of buffers, which are partially neutralized acids that resist changes in pH.
In trying to obtain a target pH—while still achieving a desired degree of sourness—various tactics can be taken.
Maintaining sourness while raising pH.
To increase the pH without reducing sourness, select buffer salts and/or acids with maximum sourness at the target pH. For example, at pH levels between 4.0-5.0, add sodium or potassium citrate to raise pH and replace part of the citric acid with malic acid to maintain sourness.
Increasing sourness while maintaining pH.
Increase the concentration of the buffer salt and the acidic ingredient, but maintain the same ratio. The pH remains close to the same, but sourness increases because there is more undissociated acid present. For example, if a formulation has a pH of 4.4 with 0.06% sodium citrate and 0.13% lemon juice concentrate, increase the sodium citrate and lemon juice concentrate levels to 0.09% and 0.195%, respectively.
Lowering pH with minimal added sourness.
At target pH levels above 4.0, use lactic acid to lower the pH with minimal added sourness. At pH levels below 4.0, use phosphoric and citric acids instead. Phosphoric acid is much less sour than citric acid but is more difficult to handle. There are patents covering the use of phosphoric acid in salad dressings and the use of glucono-delta-lactone (GDL) to lower pH without increasing sourness.
Technical contact: Daniel Sortwell, 336-712-9804, drs@usa.net
Sales contact: David Tapajna, 905-662-1127, dtapajna@yahoo.com
www.bartek.on.ca Bartek Ingredients Inc. WRITE IN 206